40 fda structured product labels
DailyMed - FDA Resources: SPL, Other Prescription Drug Labeling ... Structured Product Labeling (SPL) is the standard format for electronic submission of the content of labeling. For SPL resources (including industry data standards for SPL), see FDA's SPL Resources page and the "Structured Product Labeling Resources" heading on FDA's Prescription Drug Labeling Resources page. DailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.Instead, these archives have been split into multiple parts. The remainder archive files consist of bulk ingredient labels, vaccine labels, and some labels for medical …
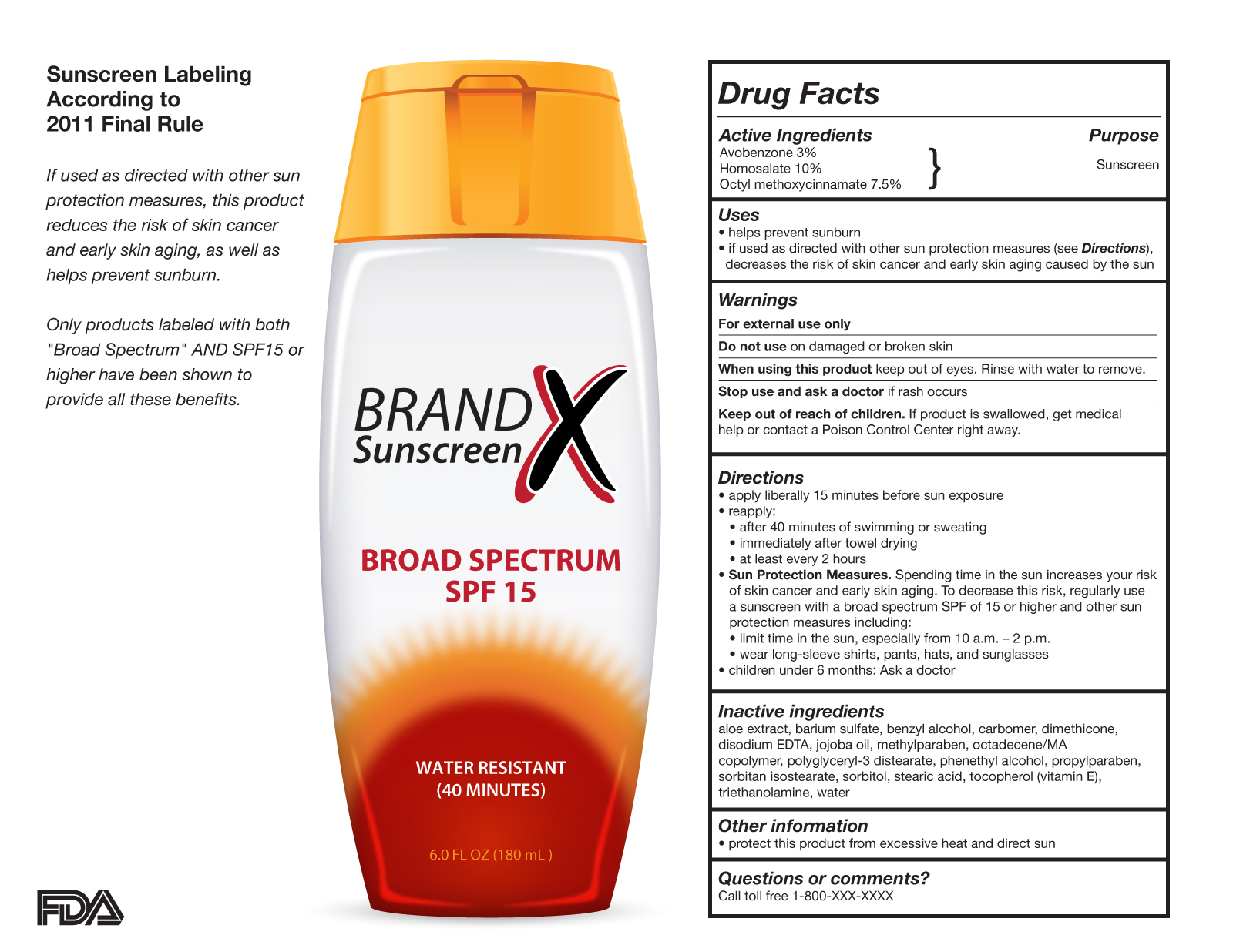
FDA Label Search The device labeling has been reformatted to make it easier to read but its content has not been altered nor verified by FDA. The device labeling on this website may not be the labeling on...

Fda structured product labels
Federal Register :: National Bioengineered Food Disclosure Standard Dec 21, 2018 · Section 66.3(a) requires that labels for bioengineered food must bear a BE disclosure consistent with the requirements of part 66. Section 66.3(a)(2) prohibits labels for food that is not bioengineered from bearing a BE disclosure unless the food may bear a voluntary disclosure under § 66.116, based on records maintained under § 66.302. NSDE | FDA - U.S. Food and Drug Administration Mar 31, 2022 · With the exception of the billing unit data in the NSDE document, this file is generated from SPL documents sent to FDA for inclusion in the FDA Online Label Repository at labels.fda.gov. Introduction to FDA Structured Product Labeling - SPL R4 SPL. The Structured Product Labeling (SPL) specification is a document markup standard that specifies the structure and semantics for the regulatory requirements and content of the authorized published information that accompanies any medicine licensed by a national or international medicines licensing authority. UCUM.
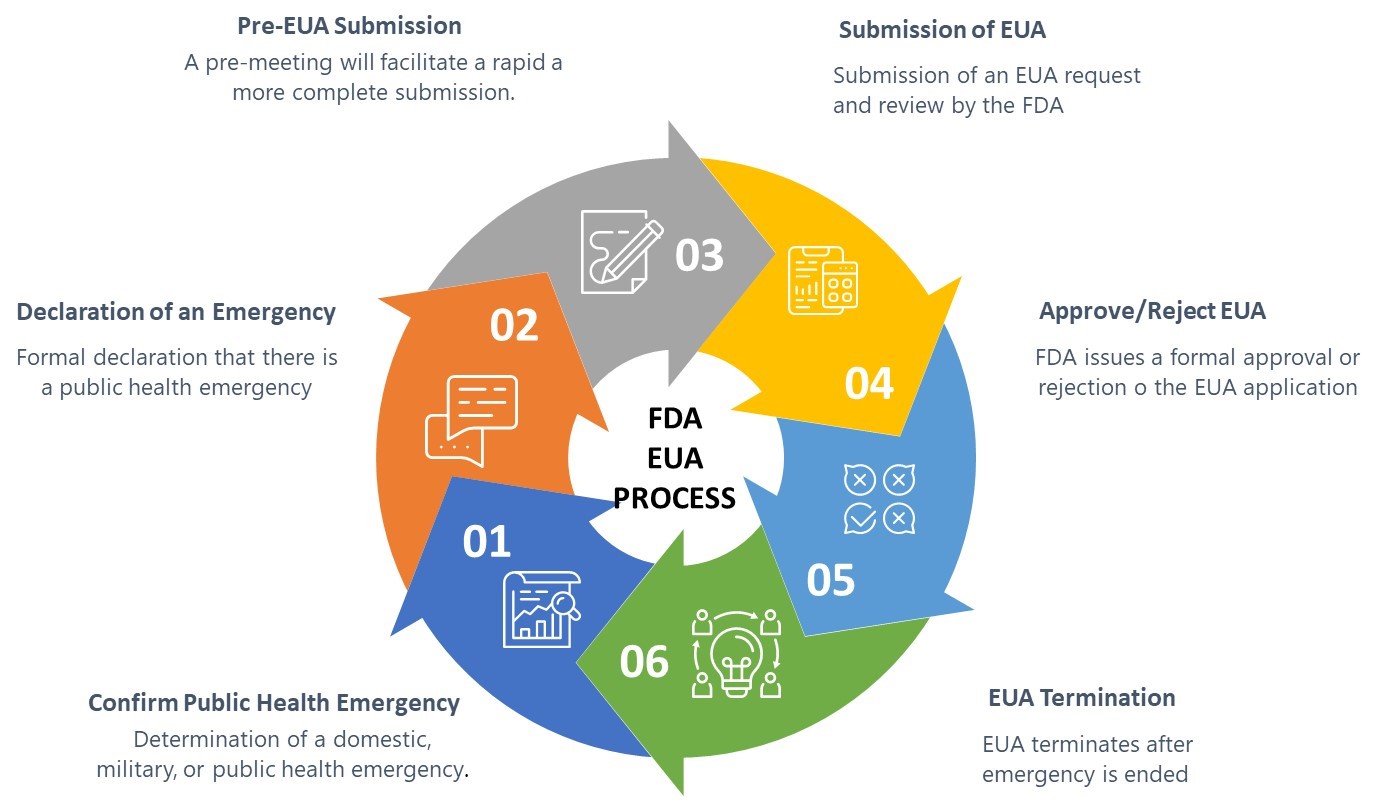
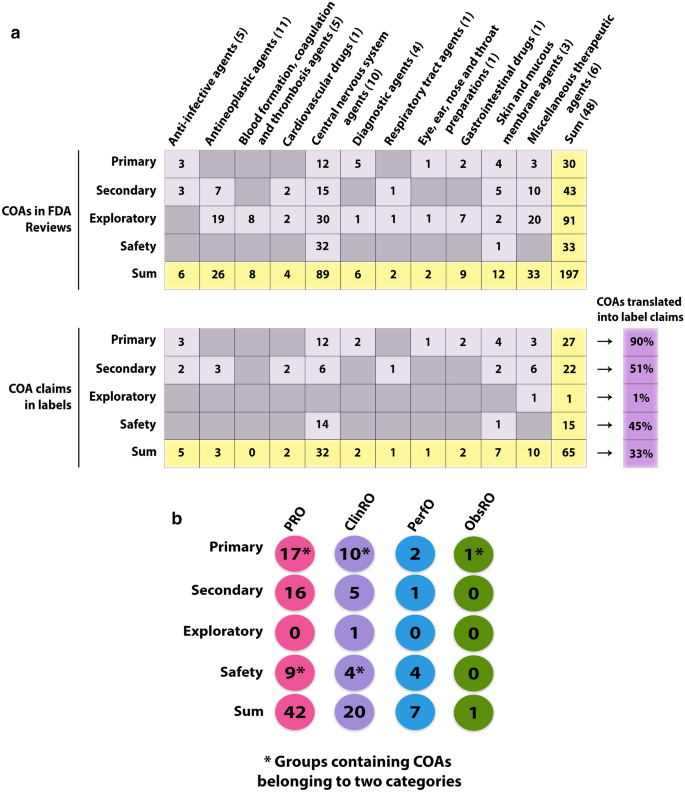
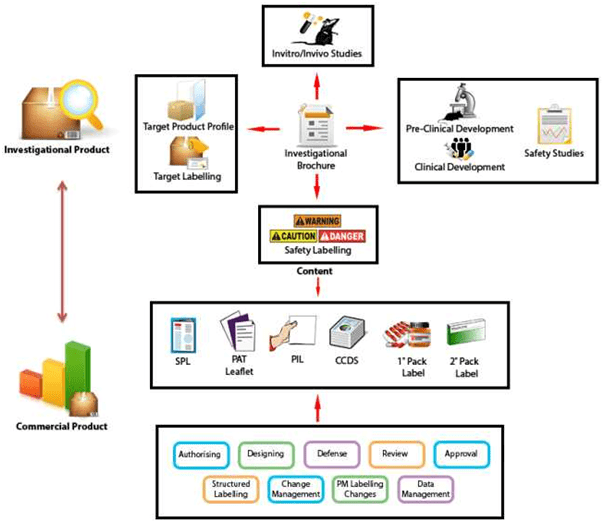
Fda structured product labels. CDER Small Business and Industry Assistance (SBIA) Learn | FDA Sep 29, 2022 · FDA Product-Specific Guidances: Lighting the Development Pathway for Generic Drugs ... Nonproprietary Name Suffix and Safety for Product Design and Labels (10/15) REdI ... Structured Product ... Assessing the Impact of HL7/FDA Structured Product Label (SPL) Content ... To understand the impact of SPL labels for current drug knowledge management and CPOE system implementation, this paper investigates (1) if SPL labels are sufficient as an exclusive source for drug information for e-prescribing systems today; (2) if SPL labels can be used directly in conjunction with other knowledge sources. FDA SPL - Structured Product & Drug Labeling Composition Process | Reed ... Structured product labeling for both prescription and over-the-counter (OTC) drugs must incorporate an overview of the scientific information needed for the correct and effective use of the drug. The labeling is broken up into sections including explanations for use (prescription drugs) or purpose (OTC drugs), adverse effects, and more. Structured Product Labeling - Wikipedia Structured Product Labeling ( SPL) is a Health Level Seven International (HL7) standard which defines the content of human prescription drug labeling in an XML format. [1] The "drug labeling" includes all published material accompanying a drug, such as the Prescribing Information which contains a great deal of detailed information about the drug.
MTHSPL (FDA Structured Product Labeling) Source Information Authority The U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug Administration (FDA) Structured Product Labeling (SPL). Information for this source is extracted from the NLM DailyMed Web site. Purpose Structured Product Labeling Resources | FDA Aug 17, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ... MTHSPL (FDA Structured Product Labels) - Statistics FDA Structured Product Label imprint attribute for shape text: 18077: BLA: Therapeutic Biologic Applications number for the MTHSPL drug: 15324: NDA: New Drug Application number for MTHSPL drug: 11751: DCSA: Controlled Substance Act designation code (e.g. 0,2,3n) 7193: MARKETING_EFFECTIVE_TIME_HIGH:
Structured Product Labeling - Food and Drug Administration Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. The... Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. PPIC Statewide Survey: Californians and Their Government Oct 27, 2022 · Key Findings. California voters have now received their mail ballots, and the November 8 general election has entered its final stage. Amid rising prices and economic uncertainty—as well as deep partisan divisions over social and political issues—Californians are processing a great deal of information to help them choose state constitutional officers and … Indexing Structured Product Labeling | FDA Center for Drug Evaluation and Research Center for Biologics Evaluation and Research This guidance explains that FDA's Center for Drug Evaluation and Research (CDER) and Center for Biologics...
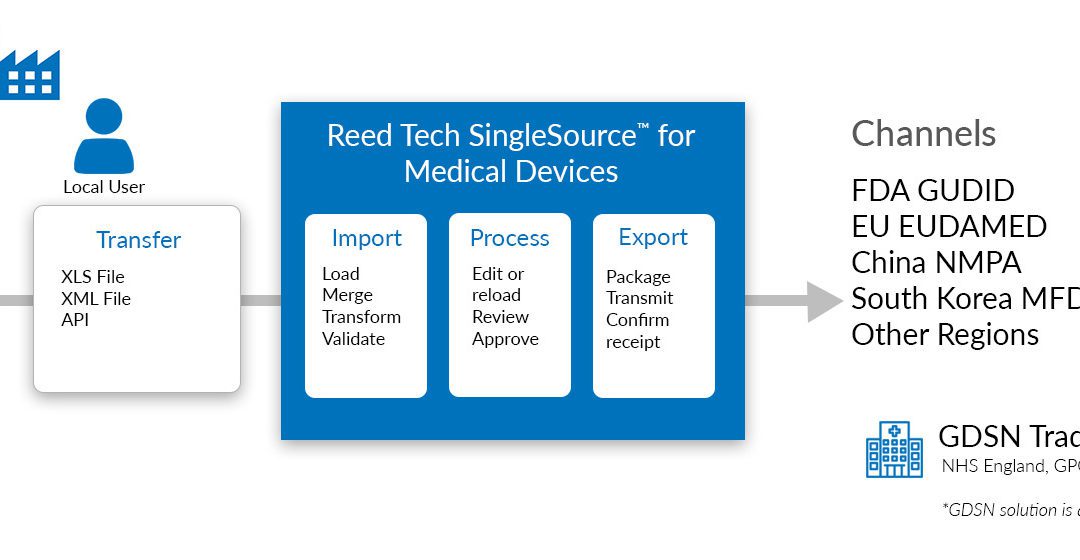
Structured Product Labeling (SPL) | Data Conversion Laboratory - DCL Structured Product Labeling (SPL) is a standard used by the FDA community to facilitate the communication of drug labeling data reliability among various groups such as the FDA, hospitals, prescribing organizations, doctors, and the general public. SPL is an HL7 and ANSI approved standard.
August 23, 2021 Approval Letter - Comirnaty - Food and … Aug 23, 2021 · the final content of labeling (21 CFR 601.14) in Structured Product Labeling (SPL) format via the FDA automated drug registration and listing system, (eLIST) as described at
SPL for FDA Submission - Dakota Systems Through the use of a standard, structured format, measurable improvements can be achieved throughout the creation, review, approval and overall management and distribution of labeling content by both industry and health authorities. ... SPL Challenges and FDA Compliance. Product labeling is a highly regulated and complex process. The product ...
Radio-frequency identification - Wikipedia Radio-frequency identification (RFID) uses electromagnetic fields to automatically identify and track tags attached to objects. An RFID system consists of a tiny radio transponder, a radio receiver and transmitter.When triggered by an electromagnetic interrogation pulse from a nearby RFID reader device, the tag transmits digital data, usually an identifying inventory number, …
Drug Labeling Overview - Food and Drug Administration Drug manufacturers and distributors submit documentation about their products to FDA in the Structured Product Labeling (SPL) format. The openFDA drug product labeling API returns...
Amiloride: Uses, Interactions, Mechanism of Action - DrugBank with evidence-based and structured datasets. See how. Build, train, & validate predictive machine-learning models with structured datasets. ... Drug product information from 10+ global regions. Our datasets provide approved product information including: ... FDA label. Download (377 KB) MSDS. Download (39.3 KB) Clinical Trials Clinical Trials ...
Introduction to FDA Structured Product Labeling - SPL R4 SPL. The Structured Product Labeling (SPL) specification is a document markup standard that specifies the structure and semantics for the regulatory requirements and content of the authorized published information that accompanies any medicine licensed by a national or international medicines licensing authority. UCUM.
NSDE | FDA - U.S. Food and Drug Administration Mar 31, 2022 · With the exception of the billing unit data in the NSDE document, this file is generated from SPL documents sent to FDA for inclusion in the FDA Online Label Repository at labels.fda.gov.
Federal Register :: National Bioengineered Food Disclosure Standard Dec 21, 2018 · Section 66.3(a) requires that labels for bioengineered food must bear a BE disclosure consistent with the requirements of part 66. Section 66.3(a)(2) prohibits labels for food that is not bioengineered from bearing a BE disclosure unless the food may bear a voluntary disclosure under § 66.116, based on records maintained under § 66.302.








.png.aspx)

















Post a Comment for "40 fda structured product labels"